Bond Angle of Cyclohexane
And you can see that this has a carbon-carbon double bond and so does this molecule. If we drew out a ring like this we already know this is called cyclohexane.

Cyclohexane Conformations Teaching Chemistry Organic Chemistry Study Organic Chemistry Books
What are the Uses of Saturated Hydrocarbons.
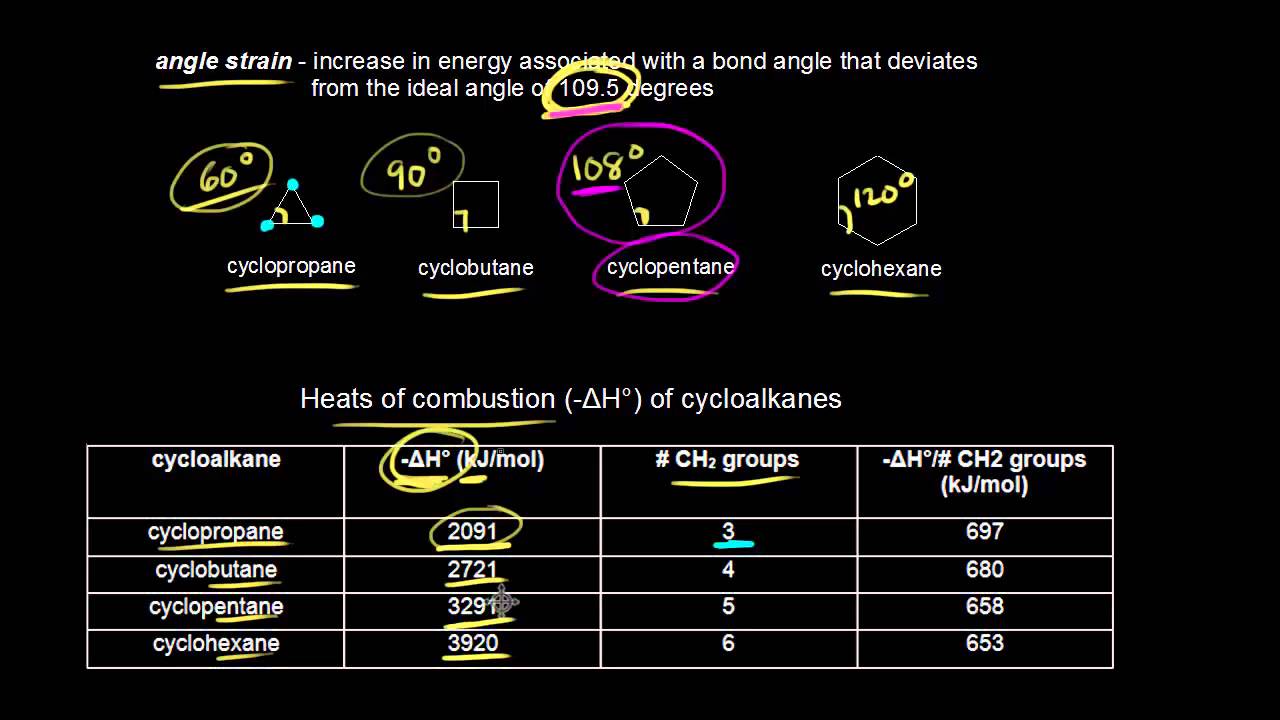
. Therefore we can define it as a vertical chemical bond. Web Small rings such as three and four membered rings have significant angle strain resulting from the distortion of the sp 3 carbon bond angles from the ideal 1095º to 60º and 90º respectively. Alkanes are widely used as fuels heating oils and solvents.
This will be illustrated more clearly later. The hydrogens on adjacent carbons are also arranged in a perfect staggered conformation that makes the ring free of torsional strain as well. This angle strain often enhances the chemical reactivity of such compounds leading to ring cleavage products.
Vbar 12πckbeta question_answer Q. Web Our double bond starts at carbon one so we put a one in front here and we call this 1-butene. The front atom is called proximal while the back atom is called distalThis type of representation clearly illustrates the specific dihedral angle between the proximal.
Web 43 Conformation Analysis of Cyclohexane. In the chair conformation of cyclohexane all the carbons are at 1095º bond angles so no angle strain applies. Provide the proper number of H NMR signals peaks for the following compound.
Both double bonds in the central B ring are exocyclic with respect to rings A and C. Web Sight along the C2-C1 bond of 2-methylpropane isobutanea Draw a Newman projection of the most stable conformationb Draw a Newman projection of the least stable conformationc Make a graph of energy versus angle of rotation around the C2-C1 bondd Assign relative values to the maxima and minima in your graph given that an HH. The axial position is perpendicular to the plane of the ring of cyclohexane.
Structural formula shows the minimal detail that shows the arrangement of. If we put in a double bond now its cyclohexene. Web Cyclohexane C 6 H 12 Cyclopropane C 3 H 6.
Web The skeletal formula or line-angle formula or shorthand formula of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecules bonding and some details of its molecular geometryA skeletal formula shows the skeletal structure or skeleton of a molecule which is composed of the skeletal atoms. Stretching frequency of a bond is represented by the following equation. Lets look at another example of an alkene.
In the compound on the right the diene is homoannular with 4 alkyl substituents. Web Due to the minimized steric hindrance the chair conformation is the most stable structure for the cyclohexane molecule. Web We would like to show you a description here but the site wont allow us.
Web This diene group has 4 alkyl substituents labeled 1234 and the double bond in one ring is exocyclic to the other adding 5 nm for an exocyclic double bond. A few other uses of saturated hydrocarbons are listed below. Since cyclopropane has a carbon-carbon bond angle of 60 o it has the highest ring strain among all cycloalkanes.
Web PalanikkumaranPK Muthiah PhD MBA Candidate Business Development Manager Sales Account Manager I Pharma Medical Healthcare Packaging I Innovation Strategy I Sustainability I Business Analytics. Web A Newman projection useful in alkane stereochemistry visualizes the conformation of a chemical bond from front to back with the front atom represented by a dot and the back atom as a circle. The bond angle of this type of chemical bonds is usually 90 degrees.

Cyclohexane Chair Flip Summary Of How To Draw A Ring Flip Mcat Study Chemistry Student Learning

Cyclohexane Conformations Chemistry Labs Chemistry Organic Chemistry

Convert Newman Projection Of Cyclohexane To Bond Line Chemistry Lessons Chemistry Textbook Study Chemistry

Stability Of Cycloalkanes Organic Chemistry Books Organic Chemistry Chemistry
0 Response to "Bond Angle of Cyclohexane"
Post a Comment